
 |
| |||
|
|
| This Document | ||
| SummaryPlus | ||
| Full Text + Links | ||
| PDF (157 K) | ||
|
| ||
| Actions | ||
| Cited By | ||
| Save as Citation Alert | ||
| E-mail Article | ||
| Export Citation | ||
Genome Analysis
Genomic analysis of gene expression relationships in transcriptional
regulatory networks
Haiyuan Yu1,
*,
Nicholas M Luscombe1,
*,
Jiang Qian2
and Mark Gerstein , 1
, 1
1 Department of Molecular
Biophysics and Biochemistry, Yale University, PO Box 208114, New Haven, CT
06520-8114, USA
2 Wilmer Institute, Johns Hopkins
School of Medicine, Baltimore, MD 21287, USA
Available online 16 July
2003.
From merging several data sources, we created an extensive map of the
transcriptional regulatory network in Saccharomyces cerevisiae,
comprising 7419 interactions connecting 180 transcription factors (TFs) with
their target genes. We integrated this network with gene-expression data,
relating the expression profiles of TFs and target genes. We found that genes
targeted by the same TF tend to be co-expressed, with the degree of
co-expression increasing if genes share more than one TF. Moreover, shared
targets of a TF tend to have similar cellular functions. By contrast, the
expression relationships between the TFs and their targets are much more
complicated, often exhibiting time-shifted or inverted behavior. Further
information is available at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fbioinfo.mbb.yale.edu%252Fregulation%252FTIG%252F
![]()
An important question in molecular biology is how gene expression is regulated in response to changes in the environment. Previous studies have explored this by making genome-wide measurements of gene expression levels with DNA arrays [1, 2 and 3] and by searching for transcription factor (TF)-binding sites using genetic, biochemical and large-scale ChIp–chip (chromatin immunoprecipitation and DNA chip) experiments [4, 5, 6, 7, 8, 9 and 10]. Here, we integrate gene-expression and TF-binding data for Saccharomyces cerevisiae to determine the effect that regulatory networks have on the expression of targeted genes.
We compiled a yeast regulation dataset by merging the results of genetic, biochemical and ChIp-chip experiments [4, 5, 7 and 10]. It contains 7419 TF-target pairs from 180 TFs and 3474 target genes ( Table 1). Regulatory networks can be simplified into six basic motifs [9 and 10] ( Fig. 1a). Here, we focus on the single input motif (SIM), multi-input motif (MIM) and feed-forward loop (FFL) as the data for the remaining motifs are too sparse.
Table 1. Summary of transcriptional regulatory network dataset
(<1K)
Abbreviations: All, all the transcription factor–target pairs; FFL, feed-forward loop; LOD, log odds ratio; MIM, multi-input motif; SIM, single-input motif; TF, transcription factor.
(16K)
Fig. 1. Transcriptional regulatory motifs and temporal gene expression relationships. (a) The six basic regulatory motifs, where circles represent the transcription factors (TFs) and squares, targets. For the single input motif, the target gene has one TF; for the multi-input motif, target gene has multiple TFs. In the feed-forward loop, the leading TF (TF1) regulates an intermediate TF (TF2), and both regulate the target gene. In autoregulation, the TF targets itself, and in the multi-component loop, two TFs regulate each other. In a regulator chain, a set of TFs regulate each other in series. (b) The four gene expression relationships: correlated, where genes have similar profiles (co-expressed); time-shifted, where genes have similar profiles, but one is delayed with respect to the other in the cell cycle; inverted, where genes have opposing profiles; and inverted time-shifted. The local clustering method uses a dynamic programming algorithm to align the expression profiles of the genes in question. From the alignment, the method is able to determine which of the four types the relationship is and assign a clustering score measuring the significance of the relationship; for the Cho et al. dataset [11], a score of 13 or above corresponds to a relationship significant to P=2.7×10-3 (see Supplementary data at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fdownload.bmnqc.com%252Fsupp%252Ftig%252FRu230_Yu.pdf).
We obtained expression profiles of yeast genes through two complete cell cycles [11]. Between the expression profiles of pairs of genes, we used a local clustering method to calculate four types of temporal relationships [12] ( Fig. 1b): correlated, time-shifted, inverted and inverted time-shifted. To find these relationships, expression levels must be assessed over a time-course, with many measurements, at small and uniform intervals. Most available datasets do not satisfy these conditions, being only suitable for simple correlation calculations (i.e. co-expression); thus, we can only conduct detailed analysis on the cell-cycle dataset. Nevertheless, similar overall results are observed in other microarray datasets.
We use several statistics to quantify the significance of our observations. The P-value is the probability that an observation (e.g. co-expression of target genes) would be made by chance, and is calculated using the cumulative binomial distribution:
The log odds ratio (LOD) is the enrichment a particular relationship in the presence of regulation with respect to random expectation for the occurrence of the relationship:
P(relationship|regulation) is the probability of gene pairs with
certain regulatory relationship (e.g. TF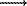 target) having a specific expression or
functional relationship (e.g. correlated expression). P(relationship) is
the probability of randomly selected gene pairs having the same expression or
functional relationship. When we report this together with P-values, we
use the following notation {log P-value;LOD value}.
target) having a specific expression or
functional relationship (e.g. correlated expression). P(relationship) is
the probability of randomly selected gene pairs having the same expression or
functional relationship. When we report this together with P-values, we
use the following notation {log P-value;LOD value}.
First, we investigate expression relationships between genes targeted by the same TFs. Overall, 3.3% of target gene pairs are co-expressed, which is four times greater than random expectation {-12;1.3} (Fig. 2a, column `All'). We detect few inverted or time-shifted relationships (see section `Effect of regulatory-signal type').
Fig. 2. Expression relationships between gene pairs. Log odds ratio (LOD) values above 0 signify observations that are more common than expected by chance, and vice versa (see Supplementary data at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fdownload.bmnqc.com%252Fsupp%252Ftig%252FRu230_Yu.pdf). (a–d) Relationships between target genes (as indicated by the color coding) for each of the different network motifs. (Note the category `All' includes all gene pairs co-regulated by at least one common transcription factor.) (a) LOD values of the likelihood that target gene pairs have correlated expression in different network motifs. (b) LOD values of the likelihood that target pairs share the same cellular function. (c) LOD values of the likelihood that target pairs with the same function have correlated expression. (d) LOD values of the likelihood that co-activated or co-repressed target pairs exhibit one of the four expression relationships. (e,f) Expression relationships between TFs and target genes. (e) LOD values of the likelihood that TFs and their target genes exhibit one of the four expression relationships in different network motifs. FFLs are divided into the TF-target relationship for the leading (TF1) and intermediate TFs (TF2). (f) LOD values of the likelihood that activator and repressor TF-target pairs exhibit one of the four expression relationships. FFL, feed-forward loop; MIM, multi-input motif; SIM, single-input motif; TF1–T FFL, TF1–target pairs in FFLs; TF2–T FFLs, TF2–target pairs in FFLs.
The level of correlation is very dependent on the type of regulatory network motif (Fig. 2a). Genes targeted by individual TFs (SIM) are not strongly correlated: just 1.3% of target pairs are co-expressed although this is significantly higher than expected {-11;0.29}. Correlation is stronger for genes targeted by multiple, common TFs: 24.4% of MIM target pairs {-12;3.2} and 5.0% of FFL targets exhibit co-expression {-12;1.6}. Similar results are observed for other expression datasets [3, 13, 14, 15, 16 and 17] ( Table 1).
The differences in enrichment (i.e. LOD values) indicate that expression is much more tightly regulated when multiple TFs are involved. However, with >100 yeast transcription factors yet to be investigated [18], unidentified TF–target relationships will probably alter the classification of SIM target genes to MIM or FFL networks in the future.
Previous studies showed that co-expressed genes tend to share similar functions [19 and 20]. By comparing the MIPS (Munich Information Center for Protein Sequences, level 2) functional classifications [21], we find that genes targeted by the same TFs are five times more likely to share functions than expected randomly {-12;1.6} ( Fig. 2b). Comparing between regulatory motifs, we again see that target genes sharing more than one common TF tend to exhibit this effect to an even greater degree (SIM{-10;1.6}, MIM{-12;2.2}). Interestingly, FFL motifs display the smallest enrichment {-11;1.5}. We speculate that this is because they have specialized effects on gene expression (see below) and so regulate a very precise subset of genes that do not necessarily share functions, but nonetheless require coordinated expression.
We also examined the expression relationships for co-targeted genes that share functions (Fig. 2c). The degree of co-expression is extremely high if targets have the same function, but low if they do not. For example, 75% of MIM target genes are co-expressed if they share functions {-12;4.3} but only 3.6% if they do not {-6;1.3}. Thus, there must be a common set of TFs for genes of similar functions to be co-expressed. Furthermore, although TFs often target genes of various functions, there are regulatory subdivisions and co-expression does not usually extend across functional categories.
We have limited experimental data describing type of regulatory signal (i.e. activation or repression) for 906 TF-target pairs [5]. Overall, target genes display correlated expression relationships (see section `Target genes are co-expressed'). However, we observe more complex relationships once regulatory-signal type is considered ( Fig. 2d). Unsurprisingly, co-activated genes mostly have correlated relationships {-12;2.3}. By contrast, co-repressed genes have a variety of relationships. The results indicate that genes activated by the same TFs co-express, but genes inhibited by the same repressors do not always co-express, although they shut down simultaneously.
Next we compared the expression profiles of TFs with their targets (Fig. 2e). Here the relationships are more complex than co-expression: SIMs exhibit time-shifted {-3;0.64} and inverted time-shifted relationships {-2;0.69}, whereas MIMs display inverted time-shifted relationships {-9;1.4}. This suggests that target genes have a delayed response to regulatory events.
FFL motifs present the most interesting and complex relationships. The leading TFs in the motif (denoted TF1) generally have negative relationships with the target genes; that is, inverted {-2;0.82} or inverted time-shifted {-10;2.0}. The intermediate TFs (TF2) exhibit all four types of relationship. The most common arrangement (55% of FFLs, Supplementary Table 2 at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fdownload.bmnqc.com%252Fsupp%252Ftig%252FRu230_Yu.pdf) is where the leading TF has a negative relationship with the target and the intermediate TF has a positive one (i.e. correlated or time-shifted). (Note, however, there are only 11 FFLs for which both TF1 and TF2 have significant expression relationships with the targets.)
As in section `Effect of regulatory-signal type', we can measure the TF–target expression relationships when the type of regulatory signals is taken into account. Although the data are too sparse to make statistically sound conclusions, we try to make some observations. Unsurprisingly, activators are co-expressed with their targets {-2;0.63} ( Fig. 2f), and comprise over 50% of TF–target pairs with significant expression relationships. We also find that repressors exhibit inverted {-2;1.1} and inverted time-shifted relationships {-2;1.2}. There are unexpected results too. Activators display significant inverted time-shifted relationships {-6;1.8} and repressors show (normal) time-shifted relationships. There are several reasons for this. A sizeable proportion of TFs (15%) act both as activators and repressors, in some cases for the same target. Furthermore, the combined effect of multiple TFs in MIM and FFL motifs can have an unpredictable effect on target expression.
In Fig. 3 we examine specific regulatory networks.
Fig. 3. Expression profiles of example regulatory networks during the cell cycle. Circles show transcription factors (TFs), squares show targets. The arrow indicates a time-shift relationship. The inset shows the relationships between the TF and target genes involved in the example. (a) Single input motif; (b) multi-input motif; (c) feed-forward loop.
Ndd1, a cell-cycle regulator during S and G2/M transition [22 and 23], acts as the sole regulating TF for Mcm21, a kinetochore protein required for normal cell growth from late S to early M phase [24 and 25], and STB5, encoding another transcription factor [26]. All three genes display cell cycle periodicity. NDD1 expression peaks early in S and sustains high expression until G2. The targets are co-expressed and time-shifted with respect to NDD1 by one time-point, peaking later in S.
Ndd1 is recruited to G2/M-transition-specific promoters by Fkh1 and Fkh2, two forkhead transcription activators [22, 23 and 27]. Collectively, these three TFs regulate Dbf2, a kinase needed for cell-cycle regulation [28], and HDR1 (function unknown). The expression profiles of the three TFs are only loosely correlated and peak at different points from early S to late G2. The targets are time-shifted with respect to FKH1 by two time-points and peak at the G2/M transition. The local clustering scores show that their expression profiles are better correlated than in the preceding SIM example (Supplementary Table 3 at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fdownload.bmnqc.com%252Fsupp%252Ftig%252FRu230_Yu.pdf).
In a feed-forward-loop, Mbp1 (a cell-cycle regulator controlling DNA replication and repair [6 and 29]) is the leading TF, Swi4 (a cell-cycle regulator controlling cell-wall and membrane synthesis [6 and 29]) is the intermediate TF, and SPT21 (a TF involved in histone expression [30]) and YML102C-A (function unknown) are the target genes. The profiles of the intermediate TF and target genes are correlated and peak sharply in G1. By contrast, the leading TF displays an inverted relationship, which highlights its involvement as a target repressor. (Previous studies have shown Mbp1 acts as an activator for ~50% its targets during the G1/S transition and as a repressor for ~10% of its targets later in the cycle [6, 7 and 29].)
In summary, we find significant connections between the networks from TF-binding experiments and gene expression data. (1) Genes targeted by the same TF are generally co-expressed and the correlation in expression profiles is highest for genes targeted by multiple TFs. (2) Genes targeted by the same TF tend to share cellular functions, and there are subdivisions within individual network motifs that separate the regulation of genes of distinct functions. (3) The expression profiles of transcription factors and their target genes display more complex relationships than simple correlation, with the regulatory response of target genes often being delayed. Note that our results are fairly robust with respect to specifics of the regulatiory network. As a check, we recalculated all our results using just the interactions in the Lee et al. dataset (106 regulators and 2416 genes) [10], and we got essentially the same results.
The datasets used for this analysis are available at http://www.sciencedirect.com/science?_ob=RedirectURL&_method=externObjLink&_locator=url&_cdi=5183&_plusSign=%2B&_targetURL=http%253A%252F%252Fbioinfo.mbb.yale.edu%252Fregulation%252FTIG%252F.
![]()
We thank Duncan Milburn for computational support. N.M.L. is sponsored by the
Anna Fuller Fund and M.G. acknowledges support from the NSF DMS-0241160. ![]()
1. M. Schena et al., Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270 (1995), pp. 467–470. Abstract-Elsevier BIOBASE | Abstract-EMBASE | Abstract-MEDLINE | Abstract-BIOTECHNOBASE
2. M. Chee et al., Accessing genetic information with high-density DNA arrays. Science 274 (1996), pp. 610–614. Abstract-BIOTECHNOBASE | Abstract-Elsevier BIOBASE | Abstract-MEDLINE | Abstract-EMBASE | Full Text via CrossRef
3. J.L. DeRisi et al., Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278 (1997), pp. 680–686. Abstract-MEDLINE | Abstract-EMBASE | Abstract-BIOTECHNOBASE | Full Text via CrossRef
4. E. Wingender et al., The TRANSFAC system on gene expression regulation. Nucleic Acids Res. 29 (2001), pp. 281–283. Abstract-EMBASE | Abstract-Elsevier BIOBASE | Abstract-BIOTECHNOBASE | Abstract-MEDLINE | Full Text via CrossRef
5. N. Guelzim et al., Topological and causal structure of the yeast transcriptional regulatory network. Nat. Genet. 31 (2002), pp. 60–63. Abstract-BIOTECHNOBASE | Abstract-EMBASE | Abstract-MEDLINE | Abstract-Elsevier BIOBASE
6. V.R. Iyer et al., Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409 (2001), pp. 533–538. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Elsevier BIOBASE | Abstract-BIOTECHNOBASE | Full Text via CrossRef
7. C.E. Horak et al., Complex transcriptional circuitry at the G1/S transition in Saccharomyces cerevisiae. Genes Dev. 16 (2002), pp. 3017–3033. Abstract-MEDLINE | Abstract-Elsevier BIOBASE | Abstract-BIOTECHNOBASE | Abstract-EMBASE | Full Text via CrossRef
8. B. Ren et al., Genome-wide location and function of DNA binding proteins. Science 290 (2000), pp. 2306–2309. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Elsevier BIOBASE | Abstract-BIOTECHNOBASE | Full Text via CrossRef
9. S.S. Shen-Orr et al., Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31 (2002), pp. 64–68. Abstract-BIOTECHNOBASE | Abstract-EMBASE | Abstract-MEDLINE | Abstract-Elsevier BIOBASE
10. T.I. Lee et al., Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298 (2002), pp. 799–804. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Elsevier BIOBASE | Abstract-GEOBASE | Full Text via CrossRef
11. R.J. Cho et al., A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell 2 (1998), pp. 65–73. Abstract-MEDLINE
12. J. Qian et al., Beyond synexpression relationships: local clustering of time-shifted and inverted gene expression profiles identifies new, biologically relevant interactions. J. Mol. Biol. 314 (2001), pp. 1053–1066. Abstract | Abstract + References | PDF (493 K)
13. A.P. Gasch et al., Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11 (2000), pp. 4241–4257. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Elsevier BIOBASE
14. A.P. Gasch et al., Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol. Biol. Cell 12 (2001), pp. 2987–3003. Abstract-BIOTECHNOBASE | Abstract-Elsevier BIOBASE | Abstract-EMBASE | Abstract-MEDLINE
15. S. Chu et al., The transcriptional program of sporulation in budding yeast. Science 282 (1998), pp. 699–705. Abstract-MEDLINE | Abstract-EMBASE | Full Text via CrossRef
16. G. Zhu et al., Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature 406 (2000), pp. 90–94. Abstract-MEDLINE | Abstract-Elsevier BIOBASE
17. P. Spellman et al., Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9 (1998), pp. 3273–3297. Abstract-BIOTECHNOBASE | Abstract-EMBASE | Abstract-MEDLINE
18. J.L. Riechmann et al., Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290 (2000), pp. 2105–2110. Abstract-MEDLINE | Abstract-BIOTECHNOBASE | Abstract-Elsevier BIOBASE | Abstract-EMBASE | Full Text via CrossRef
19. M. Gerstein and R. Jansen, The current excitement in bioinformatics-analysis of whole-genome expression data: how does it relate to protein structure and function?. Curr. Opin. Struct. Biol. 10 (2000), pp. 574–584. SummaryPlus | Full Text + Links | PDF (522 K)
20. M.B. Eisen et al., Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95 (1998), pp. 14863–14868. Abstract-MEDLINE | Abstract-BIOTECHNOBASE | Abstract-EMBASE | Full Text via CrossRef
21. H.W. Mewes et al., MIPS: a database for protein sequences, homology data and yeast genome information. Nucleic Acids Res. 25 (1997), pp. 28–30. Abstract-MEDLINE | Abstract-EMBASE | Abstract-BIOTECHNOBASE | Full Text via CrossRef
22. C.J. Loy et al., NDD1, a high-dosage suppressor of cdc28-1N, is essential for expression of a subset of late-S-phase-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 19 (1999), pp. 3312–3327. Abstract-MEDLINE
23. M. Koranda et al., Forkhead-like transcription factors recruit Ndd1 to the chromatin of G2/M-specific promoters. Nature 406 (2000), pp. 94–98. Abstract-MEDLINE | Abstract-Elsevier BIOBASE | Abstract-BIOTECHNOBASE
24. J. Ortiz et al., A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev. 13 (1999), pp. 1140–1155. Abstract-EMBASE | Abstract-BIOTECHNOBASE | Abstract-Elsevier BIOBASE | Abstract-MEDLINE | Full Text via CrossRef
25. A. Poddar et al., MCM21 and MCM22, two novel genes of the yeast Saccharomyces cerevisiae are required for chromosome transmission. Mol. Microbiol. 31 (1999), pp. 349–360. Abstract-MEDLINE | Abstract-BIOTECHNOBASE | Abstract-EMBASE | Full Text via CrossRef
26. M.M. Kasten and D.J. Stillman, Identification of the Saccharomyces cerevisiae genes STB1-STB5 encoding Sin3p binding proteins. Mol. Gen. Genet. 256 (1997), pp. 376–386. Abstract-EMBASE | Abstract-BIOTECHNOBASE | Abstract-MEDLINE | Full Text via CrossRef
27. P.C. Hollenhorst et al., Mechanisms controlling differential promoter-occupancy by the yeast forkhead proteins Fkh1p and Fkh2p: implications for regulating the cell cycle and differentiation. Genes Dev. 15 (2001), pp. 2445–2456. Abstract-BIOTECHNOBASE | Abstract-MEDLINE | Abstract-EMBASE | Abstract-Elsevier BIOBASE | Full Text via CrossRef
28. N. Grandin et al., The Cdc14 phosphatase is functionally associated with the Dbf2 protein kinase in Saccharomyces cerevisiae. Mol. Gen. Genet. 258 (1998), pp. 104–116. Abstract-BIOTECHNOBASE | Abstract-Elsevier BIOBASE | Abstract-EMBASE | Abstract-MEDLINE | Full Text via CrossRef
29. C. Koch et al., A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science 261 (1993), pp. 1551–1557. Abstract-EMBASE | Abstract-MEDLINE | Abstract-BIOTECHNOBASE
30.
C. Dollard et al., SPT10 and SPT21 are required for transcription of
particular histone genes in Saccharomyces cerevisiae. Mol. Cell.
Biol. 14 (1994), pp. 5223–5228. Abstract-BIOTECHNOBASE
| Abstract-Elsevier
BIOBASE | Abstract-MEDLINE
| Abstract-EMBASE
![]()
* These authors contributed equally to this work.
|
| ||||||||||||||||||||||||||||||||||
Volume 19, Issue 8 , August 2003 , Pages 422-427 | ||||||||||||||||||||||||||||||||||
|
Send feedback to ScienceDirect |